- Zenith Epigenetics will collaborate with Astellas Pharma on a treatment for metastatic castration resistant prostate cancer
- The companies will evaluate ZEN-3694, Zenith’s BET inhibitor, in combination with Astellas and Pfizer’s androgen receptor inhibitor, XTANDI, as a possible treatment
- The clinical study is expected to start in Q2 2021
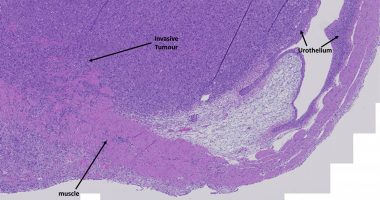
- Prostate cancer is the fifth most common cause of male cancer death worldwide
- Zenith Epigenetics is a clinical-stage biotechnology company focused on novel treatments for cancer and other disorders with unmet medical needs
- Zenith Epigenetics is a wholly-owned subsidiary of Zenith Capital
Zenith Epigenetics will collaborate with Astellas Pharma on a treatment for metastatic castration-resistant prostate cancer (mCRPC).
The companies will evaluate ZEN-3694, Zenith’s BET inhibitor, in combination with Astellas and Pfizer’s androgen receptor inhibitor, XTANDI (enzalutamide), as a possible treatment.
Zenith will sponsor and conduct a Phase 2b randomized study to evaluate the inhibitor combination relative to single agent enzalutamide in mCRPC patients who have progressed on a prior androgen receptor signaling inhibitor (ARSi).
Astellas will supply enzalutamide for the trial.
Zenith will retain all rights to ZEN-3694.
The clinical study is expected to start in Q2 2021.
Prostate cancer is the second-most commonly diagnosed cancer among men and the fifth-most common cause of male cancer death worldwide.
Adenocarcinoma of the prostate is dependent on androgen for tumor progression and depleting or blocking androgen action has been a mainstay for over six decades.
Although tumors are often initially sensitive to decreasing levels of testosterone, prostate cancer treatment has evolved rapidly over the past ten years with second-generation ARSIs.
Despite these advances, many patients with mCRPC fail or develop resistance to existing treatments, leading to low survival rates.
Donald McCaffrey, President and CEO of Zenith Epigenetics, commented,
“Data from our recently executed single arm Phase 1b/2a study has shown that the aforementioned combination may prolong radiographic progression-free survival in mCRPC patients, and particularly in a subset of patients who had a poor response to prior ARSi and whose tumors had low androgen receptor signaling activity.
There is a significant unmet need in mCRPC patients who are ARSi resistant and their current treatment options only include chemotherapy with considerable side effects. We are looking forward to validating our initial clinical findings with this well-designed randomized study.”
Zenith Epigenetics, a wholly-owned subsidiary of Zenith Capital, is a clinical-stage biotechnology company focused on novel treatments for cancer and other disorders with unmet medical needs.
Zenith Capital is a biotechnology investment company originally spun out of Resverlogix (RVX) in 2013.