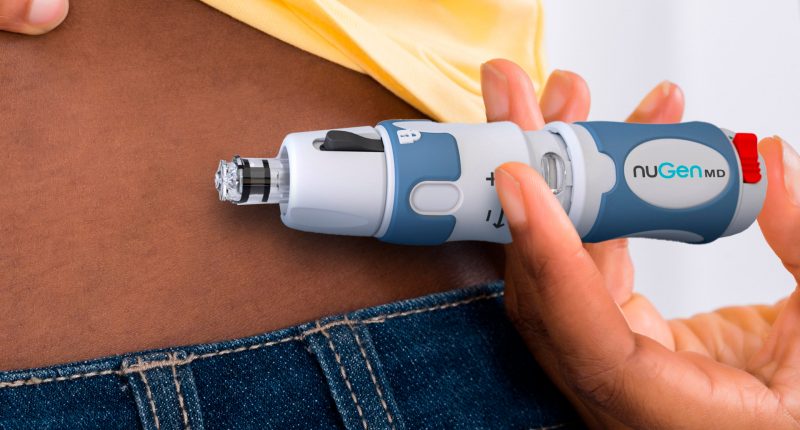
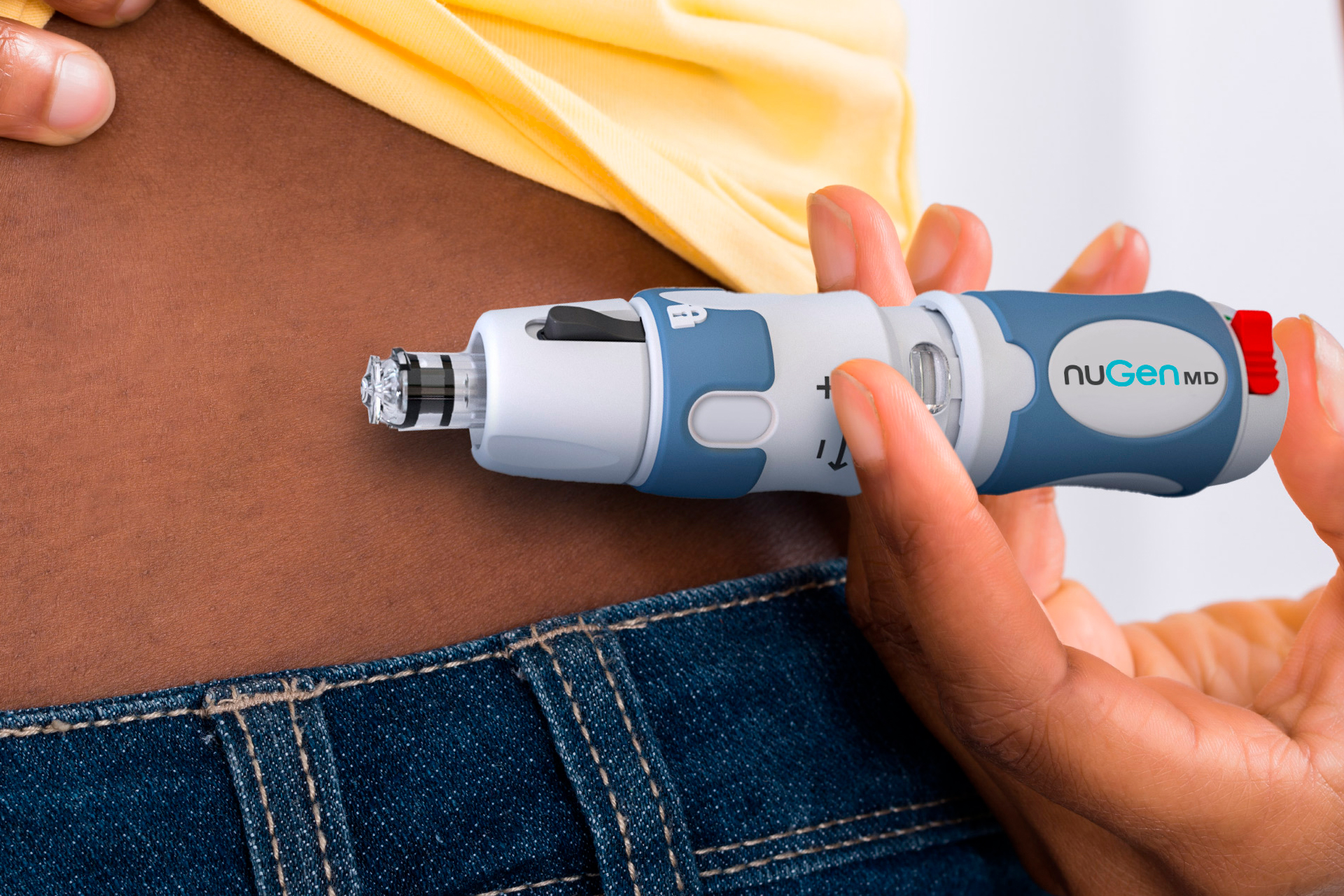
- NuGen’s (NGMD) InsuJet has been approved by Health Canada for needle-free insulin delivery
- The InsuJet needle-free injection system can be safely used 5,000 times without risk of injury or cross-contamination
- The device is approved in over 40 countries
- NuGen is a specialty medical device company
- NuGen Medical Devices (NGMD) is up by 9.52 per cent trading at $0.115 per share
NuGen’s (NGMD) InsuJet has been approved by Health Canada for needle-free insulin delivery.
The news follows last year’s general use approval from Health Canada.
The InsuJet needle-free injection system can be safely used 5,000 times without risk of injury or cross-contamination. It is approved in over 40 countries across the globe.
According to Diabetes Canada, there are more than 5.7 million people in the country living with diabetes.
“This is another important milestone in paving the way for diabetics in Canada to be able to easily acquire and use our needle-free injection device to deliver pain-free and fear-free insulin,” said Michael Wright, CEO of NuGen.
“This additional Health Canada approval brings more clarity to the intended use of the device and will further support our marketing initiatives to the diabetic community,” he added. “Our goal is to have our technologies accessible not only to the diabetic community, but to give all patients that are currently burdened with injecting themselves with a needle the option of using a needle-free device.”
NuGen is a specialty medical device company developing needle-free technologies and other innovative medical delivery products.
NuGen Medical Devices (NGMD) is up by 9.52 per cent trading at $0.115 per share as of 12:37 pm EST.