It can be hard to wrap your head around the number of diseases and conditions that people live with every day, but almost everyone knows someone that is affected by heart disease.
Around 73,000 people in the United States live with acute myocarditis and approximately 38,000 live with recurrent pericarditis, but there are several intriguing medical plays that are advancing unique treatments, and one among those that is clearly ahead of the pack.
Myocarditis is inflammation of the heart muscle (myocardium) that can reduce the heart’s ability to pump blood and pericarditis is the swelling and irritation of the thin, saclike tissue surrounding the heart.
Both can cause sharp chest pain, shortness of breath, and rapid or irregular heart rhythms. Many viruses have been linked to myocarditis, including those that cause the common cold (adenovirus); COVID-19; hepatitis B and C; parvovirus.
Focusing on this area of medicine is a clinical-stage life sciences company, Cardiol Therapeutics Inc. (TSX:CRDL).
The Company is engaged in the research and clinical development of cannabidiol (CBD) as an anti-inflammatory and anti-fibrotic therapy for the treatment of cardiovascular diseases (CVD). Its lead product candidate, CardiolRx™, is a pharmaceutically produced oral CBD formulation that is currently being evaluated in a Phase II/III multi-national study, the LANCER trial. The trial is designed to evaluate the efficacy and safety of CardiolRx™ as a cardioprotective therapy to reduce major cardiovascular and respiratory events in patients hospitalized with COVID-19 who have a prior history of, or risk factors for, CVD, and to investigate the influence of CardiolRx™ has on key biomarkers associated with heart disease.
The LANCER trial came about because of circumstances falling out from the COVID-19 pandemic. It will provide valuable information for the Company’s focus – inflammatory heart disease.
It is the Company’s most advanced trial since last year and the team has expanded it into Brazil and Mexico. Cardiol has also modified the protocol such that initially when they were only enrolling non-vaccinated patients, now vaccinated patients can participate in the trial, plus other parameters that have been included to further increase the potential patient pool.
Cardiol has also received Investigational New Drug (IND) authorization from the U.S. Food and Drug Agency (FDA) to conduct clinical studies to evaluate CardiolRx™ in two orphan drug indications:
One is a Phase II multi-national trial in acute myocarditis, expected to commence imminently, and the other is a multicenter Phase II open-label pilot study in recurrent pericarditis, to run in parallel.
The Phase II multi-national trial:
Acute myocarditis is an acute inflammatory condition of the myocardium, characterized by inflammation of the heart muscle, which may result in chest pain, impaired cardiac function, atrial and ventricular arrhythmias, and conduction disturbances.
Given the risk of significant heart failure associated with acute myocarditis, current intervention includes drugs commonly administered for heart failure. However, no generally accepted treatment exists for acute myocarditis.
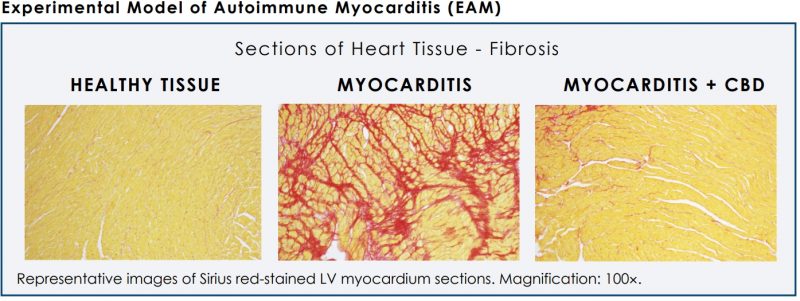
Compelling evidence has already been published that supports CBD’s anti-inflammatory and anti-fibrotic properties, promoting its use for inflammatory heart disease.
A publication in the Journal of the American College of Cardiology found that CBD has properties that can reduce inflammation and protect heart function, in a model of diabetic cardiomyopathy. It was also noted to significantly decrease cardiac fibrosis (scarring of the heart muscle) in a non-ischemic model of heart failure. Data published in an issue of Molecular Medicine also found that it can reduce cardiac inflammatory cytokine levels in a model of experimental autoimmune myocarditis (inflammatory heart failure).
Cardiol believes it has the opportunity to develop its oral CardiolRx™ formulation as an orphan drug for the treatment of acute myocarditis and recurrent pericarditis.
A major cause of sudden death in children and young adults, acute myocarditis can progress to dilated cardiomyopathy and heart failure. Severe cases can lead to extensive intensive care unit visits and expensive hospital costs.
The Phase II double-blind, randomized, placebo-controlled acute myocarditis study is designed to study the safety and tolerability of CardiolRx™ as well as its impact on myocardial recovery in patients presenting with acute myocarditis. It is expected to enroll 100 patients in 20 to 30 clinical centres across North America, Latin America, Europe, and Israel.
More recently, Cardiol received IND authorization from the FDA to conduct a Phase II open-label pilot study designed to evaluate the tolerance and safety of CardiolRx™ in patients with recurrent pericarditis. The study will also assess the improvement in objective measures of disease, and during an extension period, assess the feasibility of weaning concomitant background therapy including corticosteroids, while taking CardiolRx™.
Although generally self-limited and not life-threatening, acute pericarditis is diagnosed in 0.2% of all cardiovascular in-hospital admissions and is responsible for 5% of emergency room admissions for chest pain in North America and Western Europe. Recurrent pericarditis is the reappearance of symptoms after a symptom-free period of at least 4–6 weeks following an episode of acute pericarditis. These recurrences appear in 15% to 30% of acute cases and usually within 18 months. Further, up to 50% of patients with a recurrent episode of pericarditis experience more recurrences.
Since both acute myocarditis and recurrent pericarditis are orphan diseases in the United States, CardiolRx™ is eligible for orphan drug status under the FDA’s Orphan Drug Designation (ODD) program. The ODD program provides significant incentives, including seven-year marketing exclusivity and exemptions from certain FDA fees.
Looking ahead:
Medical plays often have very elaborate timelines, but the targets are always in motion.
This year, Cardiol Therapeutics has a lot of potentially exciting milestones remaining to achieve. Once the analysis of its trials is released, it will be like the starting pistol of a race has been fired, leading to more studies and product development.
Financial position:
In terms of finances, Cardiol Therapeutics sits on more stable ground than many others in the biotech space, which is one of its biggest differentiators. Where many medical plays are often sufficiently cashed to operate to the next year, trials often take much longer, especially if it doesn’t conclude on schedule or see an unfavourable result in the end.
Cardiol has reduced this risk and is well funded to 2024, solidly supporting its research and clinic development programs.
Meet the team:
Cardiol Therapeutics is led by a top team of who’s who of inflammatory heart disease across the board.
The Company’s President and Chief Executive Officer, David Elsley, MBA came to the role having served as founder and former President and CEO of Vasogen Inc. He has more than 30 years of experience developing, financing and managing the corporate development of life sciences companies.
His time at Cardiol represents his second take at success in cardiac research and brings with him much of his same team who has been focused on treating heart failure.
Dr. Guillermo Torre-Amione, MD, Ph.D., Chairman of Cardiol Therapeutics is a board-certified cardiologist who studied under renowned Dr. Michael DeBakey, whose claim to fame is the first successful heart transplant in North America. Dr. Torre is a Professor of Cardiology at the Methodist Hospital Research Institute, Professor of Medicine at the Weill Cornell Medical College of Cornell University, and President of TecSalud. Former Chief of the Heart Failure Division and former medical director of Cardiac Transplantation at the Houston Methodist DeBakey Heart & Vascular Center.
Recently, the Company appointed Teri Loxam, MBA and Chris Waddick, MBA, CPA, CMA to its Board of Directors.
Loxam has over 25 years of experience in the pharmaceutical, life sciences, and entertainment industries. Her diverse roles included strategy, investor relations, finance, and communications.
Loxam joined Kira Pharmaceuticals in November 2021 as Chief Operating Officer and Chief Financial Officer. In this role, she oversees finance, operations, and strategic functions for the company. Prior to joining Kira, Loxam served as Chief Financial Officer at SQZ Biotech. She was instrumental in helping the company raise over $200 million in private and public funding. This includes taking the company public through an IPO on the NYSE in October 2020.
Waddick has over 30 years of experience in financial and executive roles in the biotechnology and energy industries. He has substantial knowledge of public company management and corporate governance, and in designing, building, and managing financial processes, procedures, and infrastructure.
Waddick has served as Chief Financial Officer and Corporate Secretary of Cardiol since August 16, 2018. He serves as Executive Vice President and Chief Financial Officer for a private Ontario energy company.
Waddick has also spent more than twelve years at Vasogen Inc. While serving as Chief Financial Officer and Chief Operating Officer, the company grew from a start-up to an organization employing over 250 employees. Vasogen went public on the TSX and the NASDAQ, reaching a market capitalization of over US$1 billion.
In a news release, Chairman Torre commented on the appointments.
“We are delighted that Teri and Chris have agreed to join our Board of Directors. Their extensive and diversified experience will be invaluable to our continued growth and success as we advance the development of important new anti-fibrotic and anti-inflammatory therapeutics for the treatment of heart disease.”
Also serving on the board of directors is Colin G. Stott, BSc (Hons), Chief Operating Officer of Alinova Biosciences Ltd. He has three decades worth of experience in pre-clinical and clinical development, with specific expertise in the development of cannabinoid-based medicines.
He was the former Scientific Affairs Director, International and R&D Operations Director for GW Pharmaceuticals plc, a world leader in the development of cannabinoid therapeutics. A world authority on cannabidiol, he was largely responsible for the success of Epidiolex® and strongly feels that, whereas Epidiolex® was focused on neuroinflammation, he believes that inflammation, at the heart is, is something that he could be treating.
Investment summary:
There is a lot happening at Cardiol Therapeutics to energize investor excitement. Looking at its intellectual property, the Company boasts a comprehensive portfolio that covers and protects the formulation of CardiolRx™ as well as its formulations that target indications of heart disease.
When you look at a company like Cardiol, its main formulation being used for its clinical trials, CardiolRx™, consists of a molecule, CBD, that has been safely used anecdotally for thousands of years. CBD has also been FDA approved to treat rare forms of child-onset epilepsy successfully and safely in thousands of children. Virtually every mammal on earth has an endocannabinoid system in the body that is very receptive to various cannabinoids, including CBD.
The company is developing CBD formulations to reduce inflammation and fibrosis of the heart to treat diseases that impact the lives of thousands of people and their loved ones.
For the latest on the Company, visit cardiolrx.com.

