- Diagnos (ADK) receive approval from the Costa Rican regulatory agency for its Artificial Intelligence (AI) enabled Pathology Detection Systems
- This allows commercial deployment of the systems in Costa Rica
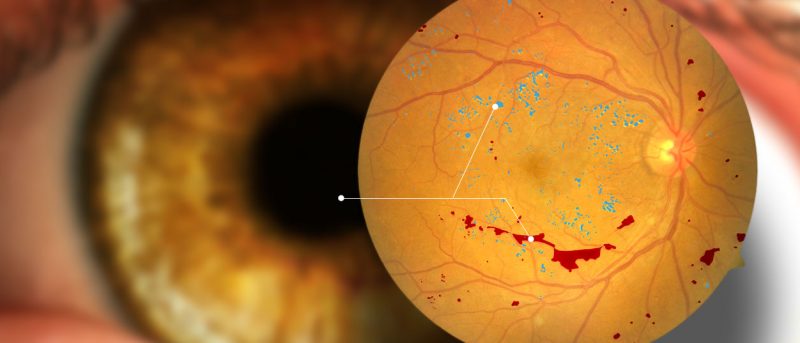
- Ocular diseases is the leading cause of blindness in the world
- Diagnos (ADK) is a Canadian corporation dedicated to early detection of critical health problems based on its FLAIRE platform based on AI
- Diagnos (ADK) unchanged today, trading at C$0.19 as of Aug 18, 2022, 1:44 pm ET
Diagnos (ADK) has received approval from the Costa Rican regulatory agency for its Artificial Intelligence (AI) enabled Pathology Detection Systems.
The company’s platform complies with legal requirements and regulations. The approval is valid for five years and allows commercial deployment of the Pathology Detection Systems in Costa Rica. The registration will be held for Diagnos by Costa Rican-based Aselcom, a local partner specializing in selling medical technologies to hospitals, medical clinics, and optical stores.
The VP of Sales, Guillermo Moreno says:
“This approval represents US$1,500,000 of potential revenues for our company.”
Ocular diseases are now the leading cause of blindness worldwide in patients between the ages of 20 and 64. In Costa Rica, 15 per cent of its population over 20 have diabetes. However, only 11 per cent of these cases are diagnosed. Of the diagnosed patients, 35 per cent were identified with diabetic retinopathy, a disease known to affect vision severely.
The President of Diagnos, Andre Larente says:
“We are very grateful to both the Quebec and Canadian Government for working with us closely on this approval for our Pathology Detection Systems in Costa Rica. This announcement reflects Diagnos’ commitment to continue to deploy our innovative Pathology Detection technology using Ocular Images not just in select countries, but worldwide.”
Diagnos (ADK) is a Canadian corporation dedicated to early detection of critical health problems based on its FLAIRE platform based on AI.
Diagnos (ADK) unchanged today, trading at C$0.19 as of Aug 18, 2022, 1:44 pm ET.