- Neovasc (NVCN) has finalised the design of the Transfemoral Trans-Septal Tiara (TF/TS Tiara) system, for treatment of the heart condition called mitral valve disease.
- The design freeze of the product now includes a modified, lower profile valve, and a steerable delivery system.
- The TF/TS Tiara is designed to be minimally invasive, self-anchoring, trackable, and fully retrievable.
- The company hopes to start a clinical study on the product’s feasibility in late 2020.
- Neovasc’s share price is down 2.77 per cent, with shares currently trading at $3.51 apiece.
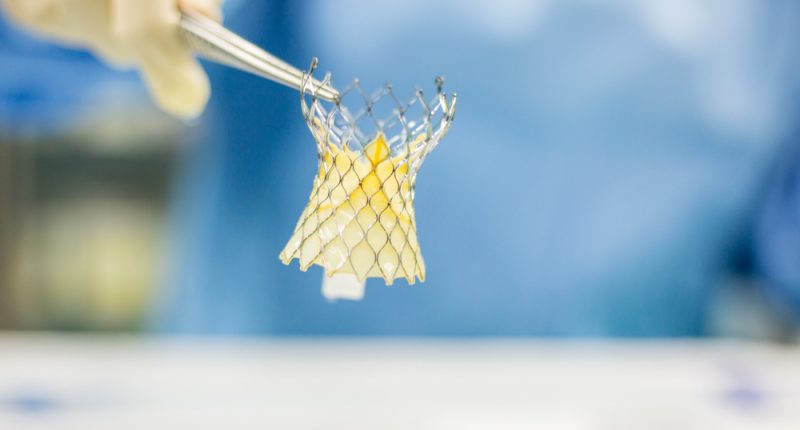
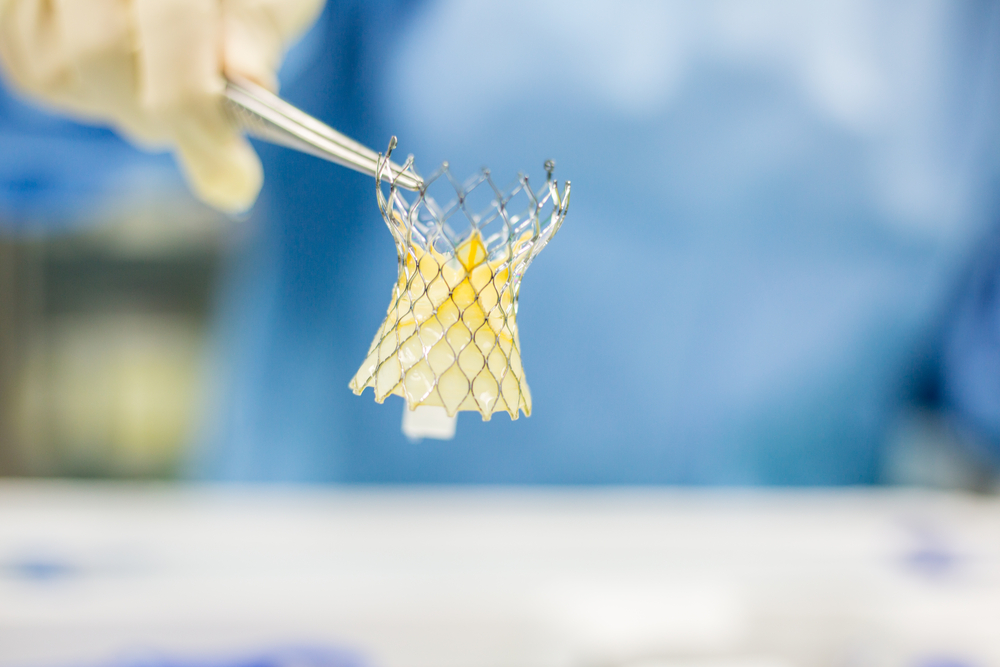
Specialty medical device company Neovasc (NVCN) has finalised the design for the Transfemoral Trans-Septal Tiara (TF/TS Tiara) system.
The Neovasc Tiara device is for treating a heart condition called mitral valve disease, or mitral regurgitation.
The design freeze for the product includes a number of changes, including a modified, lower profile valve, and a steerable delivery system. These changes may expand the pool of potential patients who are eligible for treatment.
The TF/TS Tiara system’s final design is minimally invasive, self-anchoring, trackable, and fully retrievable. President and CEO of Neovasc, Fred Colen described the product as a potential “best-in-class system.”
The Tiara is currently under clinical investigation in the United States, Canada, Israel, and Europe. Neovasc expects that they will be able to start a clinical study on the product’s feasibility in late 2020.
Neovasc’s share price is down 2.77 per cent, with shares currently trading at $3.51 apiece at 2:43pm EST.