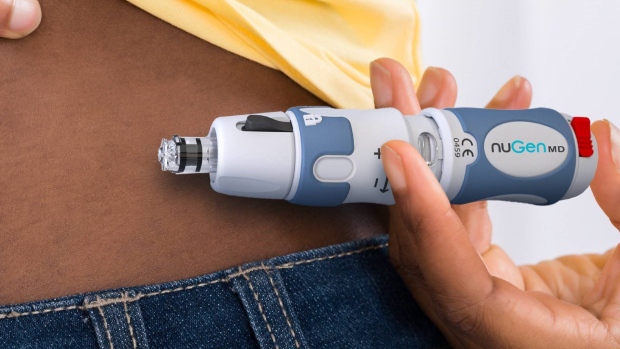
- NuGen Medical Devices (NGMD) receives approval from Health Canada for its self-administered, needle-free injection system
- The system can provide pain-free and cost-effective drug delivery
- It is currently approved in over 40 countries
- NuGen Medical Devices is an emerging specialty medical device company
- NuGen Medical Devices (NGMD) is up 16.4 per cent at C$0.39 per share at 11:43 AM EST
NuGen Medical Devices (NGMD) receives approval from Health Canada for its self-administered, needle-free injection system.
InsuJet™ is a self-administered, needle-free injection system for safe and painless drug delivery.
The system can provide pain-free and cost-effective drug delivery for millions who suffer from diabetes or other chronic illnesses.
InsuJet™ could also be used to vaccinate to protect health care workers from cross-contamination, injuries and infections.
CEO of NuGen Medical Devices, Michael Wright commented on the news.
“This is an important moment for NuGen M.D. and although our device has regulatory approval in over 40 Countries, the Health Canada approval of our needle-free device represents a significant milestone to introducing our devices to the North American market.”
It is specifically designed for health care workers and patients who are required to self-administer their medication.
Diabetes has become one of the leading causes of death worldwide in recent years.
Diabetes Canada says that complications related to diabetes are serious and can be life-threatening. The InsuJet™ can help manage blood sugar levels through daily administrations of insulin.
The InsuJet™ is currently approved in over 40 countries.
NuGen Medical Devices is an emerging specialty medical device company.
NuGen Medical Devices (NGMD) is up 16.4 per cent at C$0.39 per share at 11:43 AM EST.