- Therma Bright (THRM) reports progress on Inretio’s novel clot removal device for stroke treatment
- The PREVA device is designed to mechanically remove blood clots in the brain, a critical factor in treating ischemic stroke
- The device has been undergoing a Good Laboratory Practice (GLP) animal study
- In the future, the study is expected to be completed during the second quarter of 2023
- After which, human trials are on the docket
- It is the first and only protective clot retriever that uses a distal basket
- Therma Bright (THRM) is up under 25 per cent, trading at C$0.09 at 12:20 pm EST
Medical device technology company Therma Bright (THRM) reports progress on the development of a clot removal device with Inretio.
Inretio is a leading Israeli medical device startup developing a novel clot removal device for use in treating ischemic stroke patients.
The device, PREVA, has been undergoing a Good Laboratory Practice (GLP) animal study at the Shamir Medical Center’s Research Unit in Israel.
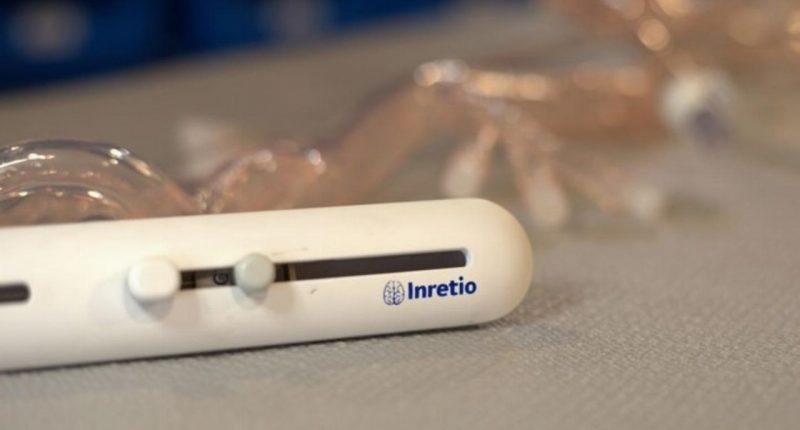
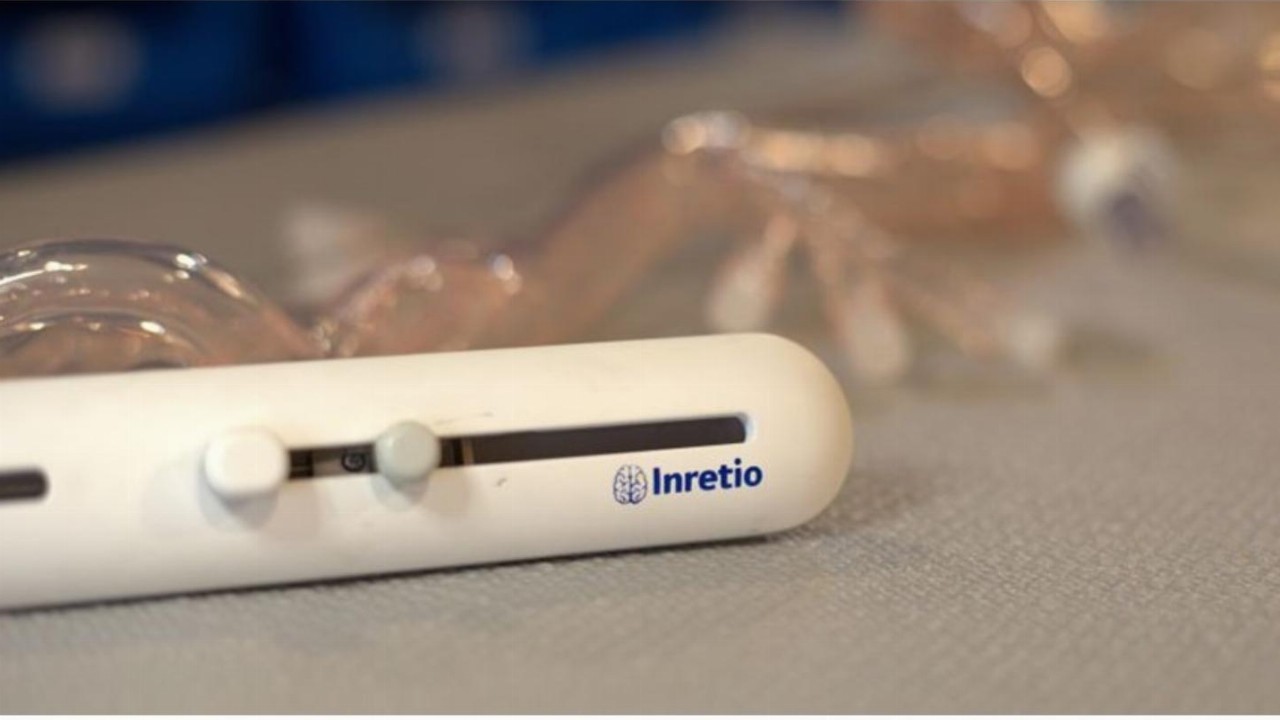
Clot removal device
According to the company, the study is moving along swiftly, and the device is able to remove 100 per cent of a blood clot in 85 per cent of cases.
Such findings are a positive sign for the device’s future development and potential regulatory approval and commercialization.
The study is expected to be completed during the second quarter of 2023.
At that specific time, Inretio plans to submit the data and mechanical verification and validation (V&V) testing data to the Israeli Ministry of Health for Clinical Trial clearance in humans.
“We are thrilled with the progress of our PREVA™ device and the positive results we’ve seen in the GLP study,” said Raviv Vine, CEO of Inretio.
“Our team has worked tirelessly to develop a device that could make a meaningful difference in the treatment of ischemic stroke, and we believe that these results bring us one step closer to achieving that goal,” concluded Vine.
How the technology works
Current mechanical solutions are based on stents that open inside the clot without aspiration. This often causes fragmentation of the clot, resulting in new blockages downstream with damage to the brain tissue.
Even a minor reduction and blood flow during the procedure can significantly impact the patient’s future quality of life.
The PREVA™ device is designed to mechanically remove blood clots in the brain, a critical factor in treating ischemic stroke.
It is the first and only protective clot retriever that uses a distal basket. Basically, it is a small net, allowing blood to flow freely through the filter.
“We are very pleased about the progress of the GLP study and share Inretio’s excitement around the potential of this life-saving ischemic stroke treatment technology,” shared Rob Fia, CEO of Therma Bright.
Positive about the work that will be done in the future, Fia carried on. “This is a serious medical issue that Inretio, in partnership with Therma Bright, looks to address with the PREVA™ device.”
Therma Bright is a developer and partner in a range of leading-edge, proprietary diagnostic and medical device technologies focused on important medical and healthcare challenges.
Therma Bright (THRM) is up under 25 per cent, trading at C$0.09 at 12:20 pm EST.