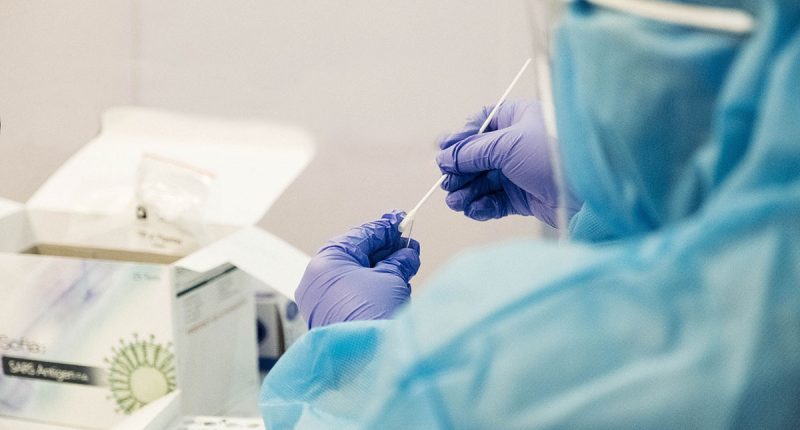
- Therma Bright (THRM) will begin its U.S. clinical performance study of its AcuVid™ COVID-19 Rapid Antigen Saliva Test after receiving conditional approval from the IRB
- The clinical performance study will begin immediately at three U.S.-based clinics
- Results will be delivered to the FDA within the next few weeks
- Therma Bright, developer of the AcuVid™ COVID-19 Rapid Antigen Saliva Test, is a progressive medical diagnostic and device technology company
- Therma Bright Inc. (THRM) opened trading at C$0.395 per share
Therma Bright (THRM) will begin its U.S. clinical performance study with the receipt of the Institutional Review Board’s (IRB) conditional approval.
The clinical performance study will begin immediately at three U.S.-based clinics. The results will complement the Brazilian clinical study and other requested test data that have been submitted to the FDA.
“We’re pleased to have received IRB conditional approval for our U.S. clinical performance study,” shared Rob Fia, CEO of Therma Bright.
“Therma Bright can begin moving forward in our efforts with three pre-selected U.S. clinics. Based on current COVID-19 infection rates, we expect the study results to be delivered to the FDA within the next few weeks, as part of our AcuVid™ application for Emergency Use Authorization (EUA).”
Since mid-July 2021, the company has actively engaged with FDA executives, doctors and scientists on its AcuVid™ COVID-19 Rapid Antigen Saliva Test application, and anticipates Emergency Use Authorization (EUA) following final FDA review.
Therma Bright, developer of the AcuVid™ COVID-19 Rapid Antigen Saliva Test, is a progressive medical diagnostic and device technology company focused on providing consumers and medical professionals with quality, innovative solutions that address some of today’s most important medical and healthcare challenges.
Therma Bright Inc. (THRM) opened trading at C$0.395 per share.