NurExone has created ExoTherapy, a cutting-edge exosome-based drug-delivery platform and is developing its lead product, ExoPTEN, as a novel therapy for acute spinal cord injuries.
Over the past two decades, exosomes – small, extracellular vesicles that are naturally released by many cell types and can carry a variety of molecular cargoes – have become the subject of increasingly intense scientific investigation. Studies suggest exosomes are important messengers for cells and organs, with as-yet unexplored diagnostic and therapeutic potential.
Today, NurExone Biologic Inc. (TSXV:NRX), headquartered in Haifa, Israel, is at the forefront of developing exosomes into next-generation nanocarriers for drug delivery. Drawing on deep expertise in exosome biology, NurExone has created the ExoTherapy technology platform, which comprises proprietary methods for the production, isolation and loading of molecules into exosomes for therapeutic purposes. NurExone is applying the ExoTherapy platform to create the company’s lead product, ExoPTEN, an intra-nasally administered exosome-based ExoTherapy to promote neuro-regeneration for the treatment of acute spinal cord injuries.
Harnessing the properties of exosomes for therapeutic applications
Many cells produce extracellular vesicles (EVs), which are organized into subtypes with different sizes and biological functions. EVs generally fall into two categories: ectosomes, which pinch off from the cell membrane by outward budding; and much smaller exosomes, which have an endosomal origin and are created when endocytotic multivesicular bodies fuse with the plasma membrane, releasing the vesicles they contain as exosomes.
Scientific understanding of the origins and functions of exosomes is advancing, but there is widespread recognition that, far from being cellular waste products as once thought, exosomes play an important biological role in intercellular communication and transmission of macromolecules between cells. Further, through their cargo-carrying capacity, exosomes facilitate the spread of proteins, lipids, mRNA, miRNA, and DNA, which can contribute to their general therapeutic effects.
Beyond their normal biological roles, exosomes have increasingly gained attention as vehicles for the delivery of active pharmaceutical ingredients (APIs), from small molecules and peptides to proteins and nucleic acids, as an alternative not only to other kinds of nanocarriers such as lipid vesicles, but also cell-based gene therapies.
Exosomes offer a number of advantages as drug-delivery vehicles. As naturally occurring biological entities harvested from cells, exosomes have completely natural membranes that are better tolerated than many other types of drug-delivery vesicles synthesized from scratch in the laboratory. At the same time, exosomes do not seem to elicit the strong immune responses that often hamper allogeneic cell-based therapies used to deliver therapeutic molecules and genes to patients. In contrast to alternative therapeutic approaches, exosome therapies do not require expensive and time-consuming personalization, but can be used as “off-the-shelf” therapies suitable for all patients.
Exosomes have additional benefits as EV-based delivery vehicles for therapeutic agents. First, exosomes, including those produced by the ExoTherapy platform, can cross the blood–brain barrier (BBB), while other nanoparticles, such as most liposomes, cannot. ExoTherapy opens up the possibility of targeting different cell types – and, by extension, therapeutic indications – that are beyond the reach of non-BBB-crossing EVs1,2. Second, unmodified exosomes, even those carrying no molecular payload, have intrinsic properties that can be therapeutically beneficial, such as anti-inflammatory effects. Finally, exosomes can be administered intra-nasally.
Exosomes originate from many sources. NurExone’s ExoTherapy platform employs exosomes derived from mesenchymal stem cells, which are effective in targeting neuronal cells. The ExoTherapy platform overcomes the many technical challenges involved in producing, purifying, and loading exosomes with APIs of almost any type (Fig. 1). Through its ability to carry a wide variety of therapeutic modalities, ExoTherapy stands as a true platform technology for creating “off-the-shelf” therapies that can be administered non-invasively.
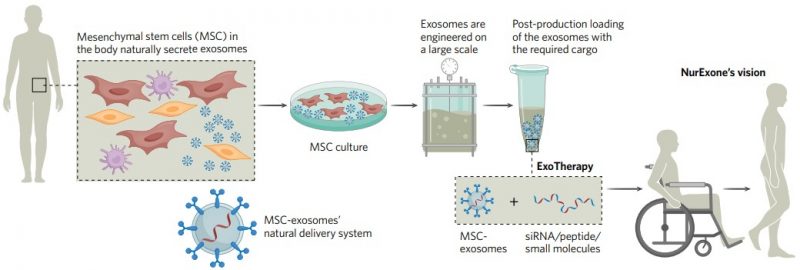
Development of a non-invasive therapy for functional recovery after SCI
The ExoTherapy platform sits at the heart of NurExone’s long-term business plan, providing a tool for creating a rich pipeline of novel therapeutic assets. In the near term, NurExone’s ambitious goal is to bring to market a novel treatment for acute spinal cord injuries (SCIs) derived from the ExoTherapy platform, ExoPTEN.
Globally, an estimated 250,000–500,000 people experience an SCI annually3, with roughly 17,000 new cases in the United States4 and 10,000 in Europe3 each year, bringing the potential market to ~50,000 new cases per year. Vehicular accidents and falls account for the majority of SCIs; sports and recreational accidents are another relatively common cause of SCI. Although the incidence of SCI is low compared with major disease such as cancer or heart disease, the effects are often devastating for patients, irreversible, and expensive to manage.
Depending on the location of the SCI, the consequences can be loss of sensory or motor control of both lower limbs (paraplegia), lower limbs and trunk, or both lower and upper limbs (tetraplegia). SCIs can also affect autonomic regulation of the body, affecting breathing, heart rate, blood pressure, temperature and bowel and bladder function.
Patients with an SCI typically spend almost two weeks in an intensive care unit, followed by a month in a rehabilitation unit. Fewer than 1% of people with an SCI experience full neurological recovery by the time of discharge, and have reduced quality of life and overall life expectancy4. SCI patients are also often frequently re-hospitalized, on average for almost three weeks, principally due to diseases of the genitourinary system, but also resulting from respiratory, circulatory and musculoskeletal problems.
In addition to the enormous physical toll SCIs exert on patients, they are also costly for health service providers. Depending on the location of the SCI, estimates place the cost of managing patients recovering from SCI at between $300,000 and >$1 million, representing a huge burden on health services and the families of new SCI patients.
There are two major obstacles to recovery from SCI. First is the poor innate regenerative capacity of the central nervous system. A major impediment to axonal growth is phosphatase and tensin homolog (PTEN), which downregulates the mammalian target of rapamycin (mTOR) activity and as a result restricts the synthesis of protein required for axonal growth. Second, SCI healing is hampered by the inflammation, myelin-associated inhibitors, glial scar components and compromised blood supply that typically surround SCIs and create a hostile environment for recovery.
ExoPTEN, which comprises exosomes loaded with small interfering RNA (siRNA) that inhibits the production of the PTEN protein, addresses both of these obstacles. The exosome component of ExoPTEN possesses intrinsic anti-inflammatory properties, which helps create a more hospitable recovery environment at the SCI site. Meanwhile, the anti-PTEN siRNA counters the suppressive effects of PTEN, activating downstream pathways necessary for the protein synthesis underlying axonal growth and regeneration (Fig. 2).
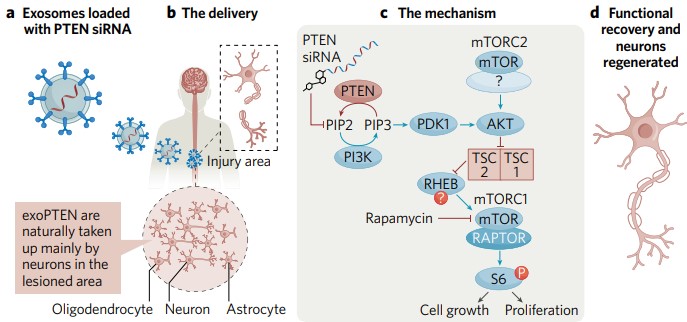
ExoPTEN has been tested as an intra-nasally administered formulation in an extreme rat model of acute SCI: complete transection of the spinal cord resulting in paraplegia. In an internal preclinical study carried out by NurExone, untreated rats remained almost totally paralyzed eight weeks after surgical transection, whereas rats receiving ExoPTEN for a maximum of two weeks showed significant partial functional recovery. No ExoPTEN human trials have yet taken place but NurExone believes the therapy could translate to improvements in quality of life. (Unloaded exosomes also demonstrated a mild effect on post-operation recovery, highlighting the dual-effect nature of ExoPTEN.) ExoPTEN also partially restored healthy electrophysiological traces, indicative of axonal rewiring and regeneration, and improved sensory recovery and urinary reflex restoration.
ExoTherapy’s applications beyond spinal cord injury
The regenerative effects observed with ExoPTEN in the severe spinal cord transection model suggest it may also have therapeutic applications in situations in which cell regeneration is a limiting factor for recovery. One major potential application of ExoPTEN identified by NurExone is traumatic brain injury, which affects more people than SCI and for which there are no effective pharmacological treatments that reduce mortality or improve functional recovery. Other potential therapeutic areas in which ExoPTEN may have a powerful impact include cardiac ischemia/reperfusion injury and associated disease, wound repair, and infertility.
While ExoPTEN employs exosomes to deliver siRNA, the ExoTherapy platform can just as easily be used to deliver other drug modalities. Moving forward, NurExone is planning to continue the development of in-house candidates such as ExoPTEN, and will also explore licensing possibilities for pharma companies looking for an enhanced delivery system for their drug(s) of various modalities, as well as opportunities to form partnerships and collaborations to jointly develop novel ExoTherapy-based medicines.
References
- Guo, S., Redenski, I. & Levenberg, S. Cells 10, 1872 (2021).
- Guo, S. et al. ACS Nano. 13, 10015–10028 (2019).
- World Health Organization. Factsheet: Spinal cord injury (2013). Available from: https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury
- National Spinal Cord Injury Statistical Center, Facts and figures at a glance. Birmingham, AL: University of Birmingham at Alabama (2016).
This is third-party provided content issued on behalf of NurExone Biologic, please see full disclaimer here.
Join the discussion: Find out what everybody’s saying about this stock on the NurExone Biologic Inc. Bullboard investor discussion forum, and check out the rest of Stockhouse’s stock forums and message boards.