Introduction
NurExone (TSXV:NRX) an innovative Canadian biopharmaceutical company, has reached a significant milestone in its efforts to revolutionize spinal cord injury treatments. The company successfully completed a pivotal preclinical study of its lead product, ExoPTEN, which demonstrated notable improvements in motor function recovery and blood flow at the injury site. This achievement brings NurExone closer to submitting an Investigational New Drug application to the FDA and initiating first-in-human trials, marking a major advancement in regenerative medicine.
ExoPTEN preclinical study
The preclinical study evaluated two dosing regimens of ExoPTEN: a single high dose administered on the day of surgery and a lower dose given over five consecutive days. Both regimens showed significant improvements in motor function recovery compared to the control group, as measured by the modified Basso, Beattie, and Bresnahan locomotor rating scale.
Histological analysis has also revealed that ExoPTEN treatment significantly increased the average blood vessel size, suggesting enhanced circulation—a critical factor in tissue healing and functional recovery.
“This preclinical study evaluated dosing regimens to provide efficacy data in support of our IND submission,” Dr. Tali Kizhner, director of R&D at NurExone said in a news release. “The results reinforce ExoPTEN’s potential to enhance the body’s natural repair mechanisms following spinal cord injury. Notably, the increased blood vessel size observed in treated subjects indicated improved circulation, which is crucial for oxygen and nutrient delivery to damaged tissues. These findings suggest that ExoPTEN has the potential to become a transformative therapeutic candidate, and we are eager to advance toward clinical trials.”
Scientific literature has long established that post-injury angiogenesis and vascular remodeling are correlated with improved functional recovery in spinal cord injury models. The positive results from this study reinforce ExoPTEN’s potential to become a transformative therapeutic candidate for spinal cord injuries.
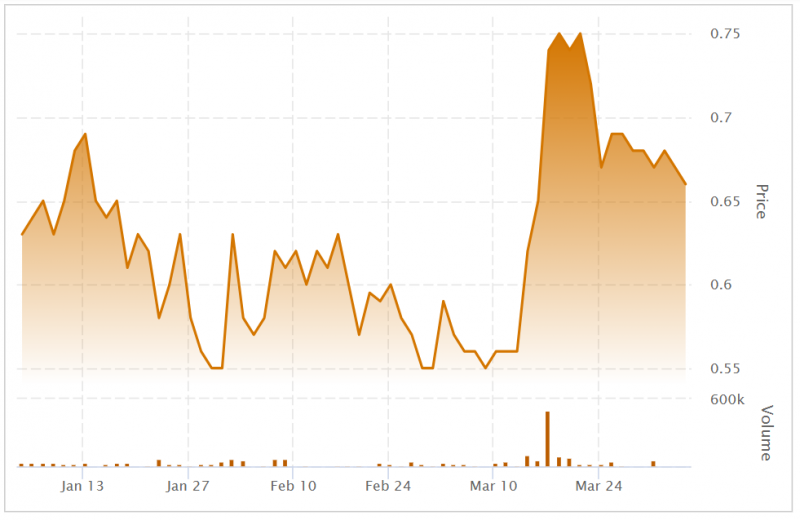
The company’s stock has risen around 10 cents since this news broke and it hasn’t looked back.
Formation of Exo-Top Inc.
In a move to bolster its production capabilities, NurExone has incorporated a U.S.-based subsidiary, Exo-Top Inc. in Nevada. This subsidiary is expected to incorporate good manufacturing practice (GMP) compliant and fully characterized exosome production. The formation of Exo-Top is a key step towards ensuring an independent and scalable supply of high-quality exosomes for NurExone’s future nanodrug pipeline and collaboration opportunities.
Exosomes are increasingly recognized as a revolutionary drug delivery system with inherent therapeutic effects. They can transport therapeutic molecules, including ribonucleic acid (RNA), proteins, and small molecules, directly to target cells with high precision and minimal invasiveness. The acquisition of a Master Cell Bank of Mesenchymal Stem Cells provides Exo-Top with a sustainable, cost-effective, and unique source of exosome-producing cells.
What is a Master Cell Bank? Company CEO, Dr. Lior Shaltiel gave an analogy to explain it in an exclusive interview with The Market Online.
“We repeatedly require glasses of milk, and the master cell bank is like an entire dairy farm,” he said. “To make this process efficient and generate revenue, we established a U.S. subsidiary. This subsidiary will be responsible for taking cells from our master cell bank—the ‘cow shed’—and producing the specific cells and exosomes we need—the ‘glasses of milk.’ Initially, this will serve our own needs, but we will also supply these products to third parties as part of a B2B business.”
C$2.3 million private placement, U.S. uplisting
NurExone recently completed a non-brokered private placement, issuing 3.5 million units at $0.65 per unit, resulting in total gross proceeds of C$2.3 million.
“This successful financing marks a significant milestone for NurExone as we expand our operational footprint and strengthen our financial position,” CFO Eran Ovadya explained in a media statement on this news. “The proceeds will be instrumental in supporting our strategic initiatives, including the establishment of a U.S. facility, which will enhance our presence in key markets and further align us with our long-term growth objectives and intent to uplist to a major U.S. exchange.”
The company plans to utilize the funds for working capital, establishing ExoTop’s U.S. production facility, and pursuing an uplisting to a major U.S. exchange. Each unit includes one common share and one warrant, with each warrant allowing the holder to purchase an additional share at $0.85 within 36 months. This transaction is pending approval from the TSX Venture Exchange.
Market opportunities and advantages
Exo-Top can not only support NurExone’s internal drug development efforts but also supply high-quality exosomes to other pharmaceutical companies, biotech firms, and researchers worldwide. This opens additional revenue streams for the company by supplying GMP-grade exosomes for drug delivery research and existing, non-U.S. Food and Drug Administration-regulated therapeutic or cosmetic applications.
The establishment of Exo-Top in the U.S. offers several strategic advantages, including proximity to strategic partners, access to a robust biopharma ecosystem, and increased market opportunities. Unlike companies that depend on third-party cell sources, Exo-Top will operate independently, without external licensing or royalty obligations, working to ensure cost efficiency and flexibility as NurExone advances its development pipeline.
Investor’s corner
NurExone Biologic Inc. is at the forefront of developing innovative treatments for spinal cord injuries and other central nervous system disorders. The successful completion of the ExoPTEN preclinical study and the strategic formation of Exo-Top Inc. position the company for significant growth and advancement towards clinical trials and commercialization. With its strong scientific foundation, strategic initiatives, and expanding market opportunities, NurExone presents a compelling investment opportunity for those looking to invest in the future of regenerative medicine.
For investors, deepening due diligence into NurExone now could provide substantial returns as the company continues to make strides in its research and development efforts, paving the way for groundbreaking therapies that address unmet medical needs.
Join the discussion: Find out what everybody’s saying about this stock on the NurExone Biologic Inc. Bullboard, and check out the rest of Stockhouse’s stock forums and message boards.
This is sponsored content issued on behalf of NurExone Biologic Inc., please see full disclaimer here.
(Top photo via NurExone Biologic Inc.)





