In 2022, over 600,000 people across the world were diagnosed with bladder cancer, a 7.1% increase from 2020, putting their life plans on hold to receive standard of care treatments that are not always effective in treating their cancer, creating an unmet need for this patient population. Cancer is generally known as an older person’s disease with bladder cancer being no exception. Approximately 80% of patients diagnosed with bladder cancer are aged 65 years or older.
If you or your loved one is a part of this group, this article is for you.
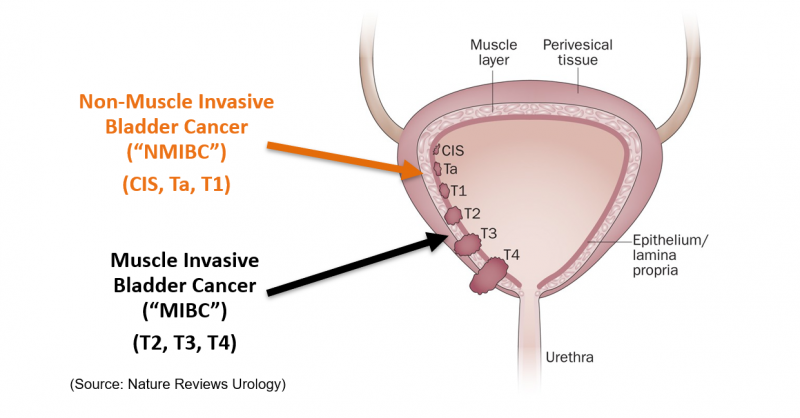
25% of bladder cancer patients are diagnosed with Muscle-Invasive Bladder Cancer (MIBC). As the name implies, the cancer has penetrated deep into the bladder wall and invaded the muscle layer. This is a condition where the standard of care may involve bladder removal (cystectomy), radiation or chemotherapy, leading to a significant reduction in quality of life.
75% of bladder cancer patients are diagnosed with Non-Muscle-Invasive Bladder Cancer (NMIBC), where: 90% have papillary disease (Ta or T1) (disease that penetrates the bladder lining, but does not penetrate the muscle layer), while 10% are diagnosed with a high-grade form of the disease known as Carcinoma In-Situ (CIS) (disease which invades the bladder lining and can spread across the surface of the bladder lining like a shag carpet).
The standard of care treatment for NMIBC is Bacillus Calmette Guérin (BCG), a bacterial immunotherapy drug originally used to treat tuberculosis that is believed to enlist a patient’s immune system to the bladder lining to destroy cancer cells.
Though remission is initially promising for 70% of patients treated with BCG, 50% of these patients have their cancers recur within one year. Along with the 30% that did not respond initially, this equates to approximately 65% of the patients treated with BCG failing treatment within 1 year and now being classified as BCG-Unresponsive NMIBC patients.
Within this BCG-Unresponsive cohort, if left untreated, approximately 40% of CIS patients risk progression to MIBC within five years – ultimately leading to bladder removal. In addition, 50% of these patients, who further progress to metastatic disease, risk losing their lives.
If you’re suffering from BCG-Unresponsive NMIBC CIS, you should be aware of the current treatments that are available today, as well as treatments that are in clinical development and may be available to you in the future, to provide you options to discuss with your uro-oncologist, preserve your bladder and with it your quality of life.
The treatment landscape for BCG-Unresponsive NMIBC CIS in 2024
For bladder cancer, there are various chemotherapy, thermo-chemotherapy and radiation treatments available.
In addition, the U.S. Food and Drug Administration (FDA) has currently approved four main drugs, with Health Canada approving two of these, to treat BCG-Unresponsive NMIBC CIS. We will discuss four other drugs currently in clinical development and vying for commercial approval in 2026 and 2027.
Let’s begin our discussion with drugs currently available in the U.S. to better understand a patient’s options in the safe and effective treatment of their disease.
FDA-approved treatments for BCG-Unresponsive NMIBC CIS
Valrubicin
Our survey begins with Endo Pharmaceuticals’ Valrubicin (Valstar), the first intravesical drug – applied directly into the bladder – approved by the FDA for NMIBC in 1981.
For patients in this clinical study, this chemotherapy-based drug achieved an initial Complete Response (CR) of 21%, but only 16.4% of these patients demonstrated a duration of that CR at one year, affording it the lowest duration of CR among competing products in the U.S. market. As a result, this drug is not generally recommended by uro-oncologists.
The least effective among approved treatments, it is also the lowest-cost at approximately US$55,000 per year. Valstar is currently unavailable in Canada.
Keytruda
The FDA approved Merck’s Pembrolizumab (Keytruda) in 2020 with a 40.6% initial CR, a 18.8% duration of CR at one year and 9.4% at two years. Health Canada approved the drug in 2024.
Keytruda is the first immunotherapy drug approved for BCG-Unresponsive NMIBC CIS, but its effectiveness is limited to patients expressing the PD-L1 protein. This reduces its applicability to approximately 20 to 40% of the BCG-Unresponsive NMIBC CIS patient population.
This drug costs approximately US$150,000 per year and requires sessions every 3 weeks for up to 24 months.
The safety profile of the drug presents an issue for both patients and uro-oncologists, as it is associated with severe adverse events, including the potential for the immune system to attack healthy organs and tissues in the body. As a result, Keytruda is generally not uro-oncologist recommended.
Adstiladrin
Ferring’s Adstiladrin became the first intravesical oncologic virus approved by the FDA for BCG-Unresponsive NMIBC CIS in 2023. Health Canada gave its approval a year earlier in 2022.
Adstiladrin’s clinical study posted a 51% initial CR, 23.5% duration of CR at one year, 18.4% at two years and 12.8% at three years.
Though its numbers are promising, the drug isn’t recommended for patients who are immunosuppressed or immunodeficient, which excludes about 6.6% of U.S. adults. Furthermore, it’s been associated with increased glucose levels and increased serum creatinine, the latter indicative of potential kidney disease.
Adstiladrin costs approximately US$211,000 per year and requires one dose every three months.
Anktiva
ImmunityBio’s (NASDAQ:IBRX) N803 drug combined with BCG, branded as Anktiva, demonstrated an initial CR of 62.3%, 59.5% duration of CR at one year and 40% at two years. It was granted the Breakthrough Designation (BTD) by the FDA in 2019 and approved for sale in 2024.
Anktiva’s strong clinical data presents two main issues for the patient and uro-oncologist:
- Firstly, combining the N803 drug with BCG, the current standard of care, raises clinical questions as to which drug is providing the efficacy. This is further hampered by the fact that, when N803 was evaluated in a similar patient population, it was unable to demonstrate effectiveness, suggesting that BCG may be responsible for a majority of the clinical effectiveness.
- Secondly, BCG is experiencing a global shortage, prompting ImmunityBio to partner with the Serum Institute of India to shore up supply, though their new strain has yet to receive FDA approval.
Anktiva is currently the most expensive FDA-approved treatment for BCG-Unresponsive NMIBC CIS on the market today, costing US$215,000 per year and requiring weekly doses over a six-week period.
Treatments for BCG-Unresponsive NMIBC CIS seeking FDA approval
EG-70
enGene’s (NDAQ:ENGN) early-stage EG-70 (detalimogene voraplasmid) is a non-viral gene therapy on track for FDA submission in 2026.
The drug uses enGene’s proprietary Dually Derivatized Oligochitosan platform, which can penetrate mucosal tissues and deliver a wide range of treatments including DNA and various forms of RNA.
EG-70 reported an initial CR of 71%, but since the phase II clinical study is ongoing, no data on duration of CR is currently available at 1 year.
Gemcitabine
Johnson & Johnson (NYSE:JNJ) has developed a slow-release form of Gemcitabine, an intravesical chemotherapy drug granted BTD status in December 2023, which is positioned for FDA approval in 2026.
The 2-to-3 minute treatment time required to install the company’s TAR-200 system into a patient’s bladder to release gemcitabine, without need for anesthesia, is attractive from both a patient’s and uro-oncologist’s point of view.
Results from phase-II trials indicate an initial CR of 83.5%, with a CR of 57.4% at one year based on a Kaplan-Meier (KM) curve (standard measure of patient survival following treatment administration).
Looming over Gemcitabine’s ultimate viability in this patient population are peer-reviewed publications reporting it to be as effective as saline at preventing progression of bladder cancer. In addition, 26.4% of patients in the clinical study discontinued treatment due to treatment-related side effects.
Cretostimogene grenadenorepvec
CG Oncology (NASDAQ:CGON) has developed an intravesical oncolytic immunotherapy, which goes by the difficult to pronounce name of cretostimogene grenadenorepvec (CG).
The drug, vying for FDA approval in 2026, demonstrated an initial CR of 74.5%, a duration of CR of 63.5% at one year and 56.6% at two years (KM Curve estimate), according to top-line phase-III data.
According to the company’s website, the drug’s mechanism of action requires that patients have a dysfunctional or negative tumor-suppressing retinoblastoma protein pathway, a result that occurs in approximately 25% of patients with high-grade NMIBC, of which CIS always is. This will ultimately limit the BCG-Unresponsive NMIBC CIS patient population that can be treated with this drug.
CG’s treatment cycle is also extensive, calling for six weekly treatments, followed by three or six weekly treatments, then three weekly treatments every three months for the next 12 months, then every 6 months for the next 24 months.
A separate phase-II study evaluating a combination treatment with Keytruda yielded encouraging results, with an initial CR of 82.9%, duration of CR of 57.1% at one year and 54.3% at two years; however, based on the reported cost of Keytruda treatments, this combinational therapy may be price-prohibitive to the patient and potentially introduce unwanted immune system attacks on the healthy organs and tissues in the patient’s body.
RuvidarTM
Theralase (TSXV:TLT), a clinical-stage pharmaceutical company dedicated to the research and development of light-activated small molecules, is developing a new drug for BCG-Unresponsive NMIBC CIS.
The company’s lead compound, the ruthenium-based molecule TLD-1433 (RuvidarTM), combines with an endogenous (naturally occurring) human glycoprotein in the body, known as transferrin, to become the patented drug, Rutherrin®, a light-activated drug that has demonstrated an ability to kill bladder cancer cells in a pre-clinical setting with over 99.9% effectiveness.
With an FDA new drug application submission planned in 2026 and potential FDA approval in early 2027, RuvidarTM has strong clinical data, achieving an initial CR of 61.9% and a duration of CR of 43.6% at one year, based on clinical data collected to date. Analyzing the KM Curve estimates, RuvidarTM demonstrated a duration of CR of 53% at one year, 35.8% at two years and 24.9% at three years in its latest phase-II results evaluating 63 patients.
What sets RuvidarTM apart from currently approved drugs and those vying for regulatory approval is its ability to completely destroy bladder cancer in 68.3% of patients (over 2 out of 3) after only one three-hour treatment. This provides a clear advantage to both the patient, who needs to attend each appointment, and the uro-oncologist or medical oncologist who needs to supervise each treatment.
Unlike a few of the drugs discussed here today, urologists can prescribe RuvidarTM across the entire BCG-Unresponsive NMIBC CIS patient population, without screening for the absence of various proteins for fear of a lack of effectiveness. In addition, RuvidarTM is used as a monotherapy (by itself) and does not need to be combined with other drugs to increase its efficacy or duration of response.
Supporting its high safety profile, the company believes that all serious adverse events in the clinical study have been unrelated to RuvidarTM or its proprietary TLC-3200 medical laser activation system.
These benefits have all eyes on Theralase®‘s ongoing phase-II clinical study, which has enrolled 75 patients to date and is on track to complete enrollment in 2025 through the recent launch of additional clinical study sites in Canada and the U.S.
The FDA awarded Theralase® the Fast-Track Designation in 2020, granting the company early and frequent communication with the federal agency. The company expects to complete patient follow-ups in 2026, with FDA and Health Canada regulatory approvals expected in early 2027.
In summary, if you are a patient or know someone diagnosed with BCG-Unresponsive NMIBC CIS, bladder removal is now not the only option on the table, as the latest approved drugs and those in clinical development have steadily increased the duration of CR over the past five years.
Talk to your uro-oncologist about how the drugs we’ve discussed in this article may be applicable to your particular cancer journey.
This is sponsored content issued on behalf of Theralase® Technologies Inc., please see full disclaimer here.
(Top photo: Adobe Stock)



