In the world of medical devices, all companies are trying to fulfill unmet needs, but only a chosen few will differentiate themselves through added efficiency above what competitors have been able to achieve.
The area of Dry Eye Syndrome is an illustrative example, with more than 340 million people affected globally, including about 40 million in the US – suffering from inflammation, nerve pain, light sensitivity, irregular tear production and potential blindness – who must undergo multiple complex, unrelated and often inconsistent tests covering different subtypes of the disease – including evaporative and aqueous deficient – increasing the chances of misdiagnosis, especially from general ophthalmologists without specialized training.
In this way, Dry Eye’s deficient treatment landscape, spanning a global patient base almost as large as the United States, is incentivizing technology companies to garner market share by innovating towards streamlined, more personalized diagnoses and ultimately more satisfactory medical outcomes.
Introducing DiagnosTear Technologies
Enter DiagnosTear Technologies (CSE:DTR), market capitalization C$45.74 million, a company playing a leading-edge role in the diagnosis of ocular diseases through its clinical-stage TeaRx platform, which has yielded early evidence in support of rapidly and reliably identifying the underlying causes of Dry Eye Syndrome in a single test – which would make it the only solution of its kind on the market – while avoiding the complexities and discomfort associated with existing standards of care.
This article is disseminated in partnership with DiagnosTear Technologies Inc. It is intended to inform investors and should not be taken as a recommendation or financial advice.
To properly convey the leadership position TeaRx lines up DiagnosTear to grow into, as well as demonstrate why the stock’s 56 per cent gain since inception in December 2024 likely represents a steep discount to future potential, let’s begin by going over the mechanics of TeaRx’s innovation, which will lead us into the company’s first product, TeaRx Dry Eye, recently approved to hit the European market, followed by its second, TeaRx Red Eye, which is rapidly accelerating on its path to pursuing a second ophthalmic revenue vertical.
How DiagnosTear’s TeaRx platform works
DiagnosTear’s TeaRx is a point-of-care technology that tests tears for target ophthalmic pathologies, deploying a multi-parameter diagnostic framework for selected applications, analyzing the results through an on-cloud smart algorithm and displaying them through visual or digital readout.
The technology opens the door for a more accurate diagnosis of ocular diseases thanks to being able to identify and segment biomarkers in the tear film associated with different diseases.
Tears are bearers of biomarkers tied to a diversity of diseases, including Dry Eye Syndrome, a variety of infective, chemical or allergic eye inflammations, and other ophthalmic conditions. Biomarkers in tears have been shown to also provide valuable information on systemic diseases such as multiple sclerosis, amyotrophic lateral sclerosis, epilepsy, Alzheimer’s disease, Parkinson’s disease and cancer, making them a key source of insight for a wide range of physicians and patient populations.
TeaRx Dry Eye
DiagnosTear’s first iteration of TeaRx comes in the form of TeaRx Dry Eye, the only test proven to detect the severity and root causes of Dry Eye Syndrome, differentiating between aqueous and evaporative variants in a single test.
The solution uses a cost-effective, non-invasive microfluidic tear collector, a muti-parametric immunochromatographic test cassette and an-on-cloud deployed interpretation algorithm which can deliver results in less than 10 minutes.
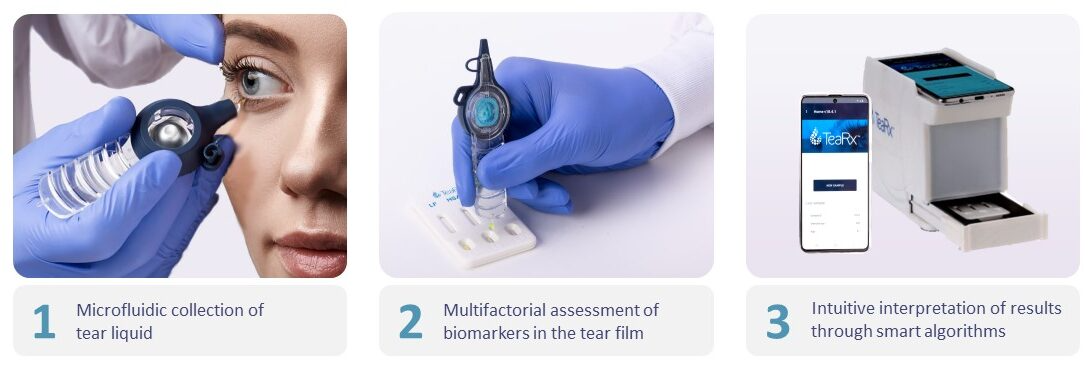
In initial clinical trials in the US and Israel, collectively including hundreds of patients, TeaRx Dry Eye showed sensitivity levels of 86 per cent and specificity levels of 87 per cent for the detection of severe Dry Eye Syndrome. In a pivotal, longitudinal study performed recently in India including 600 subjects (500 dry eye patients and 100 healthy controls), results indicated:
- More than 80 per cent sensitivity for differentiating severe vs. non-severe Dry Eye.
- More than 80 per cent sensitivity for the detection of severe Meibomian Gland Dysfunction, (the main cause for Evaporative Dry Eye) in Dry Eye patients.
- More than 92 per cent prediction rate for a patient’s responsiveness to Cyclosporine A at baseline, a potent immunosuppressant drug effective at treating Dry Eye, making it the only product capable of making this assessment.
TeaRx Dry Eye’s initial clinical success led to Conformité Européenne In-Vitro Diagnostic (CE-IVD) regulatory approval in 2024, validating that the test meets European Union (EU) health, safety and environmental protection standards.
This regulatory milestone, in turn, has allowed DiagnosTear’s leadership team, who we’ll meet later in this article, to devise and roll out an ongoing, fully funded plan to deliver TeaRx Dry Eye to strategic European markets in 2026, with eyes on leveraging the product’s first-mover treatment optimization towards greater market share, setting the stage for US deals and permitting under the US Food and Drug Administration to foster further momentum.
The company is currently in discussions with distributors across the world and expects to sign numerous deals over the next six months, making TeaRx Dry Eye – also approved by Israel’s Ministry of Health – one of the most prospective candidates to unite Dry Eye Syndrome’s diverse and distributed sub-populations and minimize the suffering of potentially hundreds of millions of people, all while delivering transformational results for DiagnosTear’s business and its investors.
TeaRx Red Eye
Despite TeaRx Dry Eye’s company-making potential, DiagnosTear is not resting on its laurels when it comes to expanding and reinforcing its long-term growth runway.
The company is fully funded to develop a second test under the TeaRx platform for Red Eye, another complex condition with numerous potential causes, each of which requires its own distinct tests under current standards of care, straining both the patient and the broader healthcare system to merely deliver a diagnosis. Here’s a breakdown of Red Eye’s major causes:
- Allergic Conjunctivitis affects about 6 million patients in the US and up to 20 per cent of the general population.
- Herpetic Keratitis comes in at 1.5 million patients globally and 500,000 in the US.
- Adenoviral conjunctivitis, for its part, affects approximately 20 million patients in the US.
- Combined, these conditions account for about 2-3 per cent of all office visits in primary care settings, as well as emergency rooms, representing a significant unmet need and value-creation opportunity.
DiagnosTear’s solution, its patented TeaRx Red Eye system, is the only test that delivers differentiated assessments for all three of these conditions, pairing a simpler, lower-cost diagnosis for the patient with triple reimbursement potential, plus the added flexibility of nurses, technicians and general practitioners being able to administer the test without a referral to an ophthalmologist.
A healthcare professional collects a sample of the patient’s tear fluid, no more than a few microliters, through an easy-to-use swab, transferring the sample into a minimal volume of assay buffer designed to maximize biomarker concentration and sensitivity, delivering results in only 10 minutes.
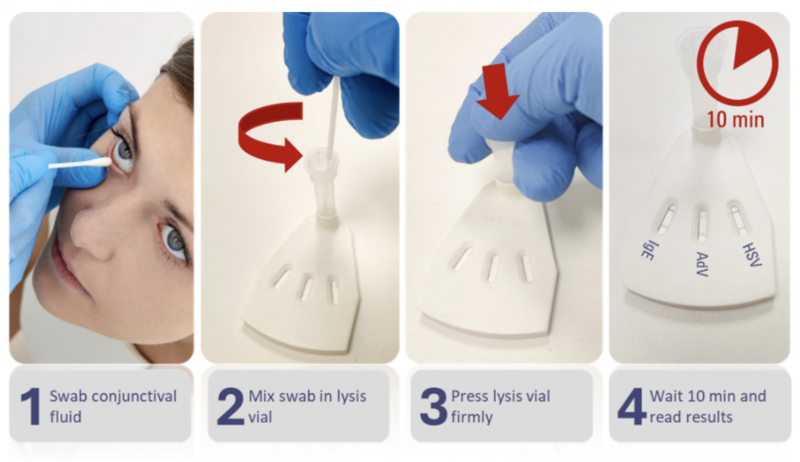
TeaRx Red Eye’s early-stage testing shows it to be a superior product compared to competitors, including QuidelOrtho’s Quickvue adenoviral conjunctivitis test, which requires aggressive brushing of the conjunctiva and is only designed to detect the Adenovirus, or Axim Biotech’s IgE test, which requires a reader, holds no CLIA Waiver and is only useful for the detection of ocular allergies. What’s more, the product adds to its value proposition by being the only point-of-care test for ocular herpes in development today.
DiagnosTear is on track to do for Red Eye what it’s on the verge of achieving for Dry Eye patients; namely, ushering diagnosis into the 21st century through a radically simplified solution.
The company has announced a clinical study with AlyaTec, a globally renowned Contract Research Organization, for testing the analytical performance of the Tear IgE (Allergy) component of the multi-parameter test kit, in a well-defined cohort of allergy patients, before and after topical provocation with an allergen, thus laying the groundwork towards CE-IVD, FDA and CLIA Waiver applications and eventual commercialization. Recent milestones in this direction include:
- Completing the plastic injection mold to produce the first 100,000 TeaRx Red Eye devices, allowing DiagnosTear to capitalize on the technology’s first-mover advantage and boost revenue as opportunities arise, while fine-tuning operations for larger-scale manufacturing.
- Partnering with the LV Prasad Eye Institute in Hyderabad, India, a top name in global eye care spanning clinical care, research and education, to provide a well-defined and characterized cohort of Ocular Herpes samples for assessing the diagnostic performance of the test. The collaboration with LV Prasad complements numerous institutional partnerships, including a Red Eye clinical trial with Israel’s largest Health Maintenance Organization, as well as Sun Pharma, a global pharmaceutical company supporting the trial with LV Prasad, each of which encapsulates DiagnosTear’s diligent approach to improving patient care, placing TeaRx Red Eye on a path to commercialization as soon as next year.
A purpose-built leadership team
DiagnosTear’s goal of elevating tear-based diagnostics into a new standard of care is in the hands of a leadership team whose robust experience in ophthalmology, IVD, medical devices and drug development solidifies conviction in TeaRx’s path to market. Let’s meet them now:
Executive management
- Dr. Shimon Gross, Chief Executive Officer (CEO), brings more than 20 years of experience in IVD, including as the former vice president of the genomics division of AID Genomics and former vice president of sales and marketing at Savyon Diagnostics, all while building a portfolio of more than 25 peer-reviewed publications and patents to his name.
- Dr. Amos Sommer, Vice President (VP) of Technology, has an almost three-decade track record in IVD R&D, and is world-renown as an expert in lateral-flow rapid tests. Sommer developed the HIV rapid test line for Alere (now Abbott), as well as the core TeaRx platform and Dry Eye Syndrome test.
- Yifftach Biel, CPA, is the CFO of BioLight (TASE:BOLT), Israel’s only pure-play ophthalmology investment company – and the 44 per cent majority shareholder of DiagnosTear – offering exposure to a portfolio focused on advanced medical devices, medication, diagnostics and digital healthcare from clinical trials to commercialization.
Board of directors
- Yaacov Michlin, Chairman, has also served as CEO of Biolight since April 2020, and previously managed a medical device company active in the US, which he ushered to an initial public offering on the Nasdaq. Michlin is a director for numerous Biolight portfolio companies and leads life sciences activities at IATI, the umbrella organization of Israel’s high-tech sector. He is also chairman of MIXIII Health Tech IL, a major conference in Israel.
- Julia Reznick Zilberman, Director, has served as VP of business development at Psifas, a national Israeli initiative for precision medicine, since May 2024. Prior to that, Zilberman led strategic initiatives at an AI drug development company from January 2023 to February 2024, and served as VP of finance and business development at an ophthalmology startup from January 2018 to January 2023, completing several funding rounds. She established her career at Teva Pharmaceutical Industries, where she spent 18 years in senior finance and commercial roles.
- Karin Gurevitz, Director, is a legal advisor with 25 years of global experience in legal and compliance management for public and private companies across numerous fields. She assumed the roles of VP, group general counsel and secretary at Biolight beginning in 2015.
- Igal Kohn, CPA, Director, took the role of CFO at medical device company Elcam, Israel’s largest medical equipment and diagnostic products manufacturer, in January 1998, managing subsidiaries and handling mergers and acquisitions. He progressed to the role of CEO in January 2017. As DiagnosTear’s second-largest shareholder, Elcam offers the company access to millions of TeaRx units in potential capacity.
- John Sinclair, CPA, Director, is a public company accounting and auditing professional with decades of experience, including serving as senior partner with audit firms Smith, Nixon LLP, Collins Barrow Toronto LLP and Baker Tilly WM LLP, managing complex projects and providing financial advisory to clients around the world. Sinclair is director of Lifeist Wellness (TSXV:LFST), an acquirer and developer of global wellness brands.
Governed by a team of executives deeply familiar with TeaRx’s target markets, regulatory pathways and go-to-market playbooks, DiagnosTear presents itself as the complete package healthcare investment, positioned to benefit from high demand and differentiated assets to fill it. This begs the question about why DiagnosTear stock has met the company’s vast promise with limited enthusiasm.
A near-term leader in its class
Despite DiagnosTear being built to capitalize on Dry Eye and Red Eye’s multi-billion-dollar testing enhancement opportunities, investors have enjoyed an only 56 per cent return since inception in December 2024, representing a steep bargain compared to the leadership position the company is on track to step into. This dislocation is likely because of factors that will sound familiar to investors accustomed to sourcing returns from market inefficiencies:
- First, DiagnosTear is pre-revenue and therefore more susceptible to higher volatility, requiring investors with a higher risk tolerance to patiently wait as leadership taps the capital markets, leveraging a mere 60.19 million shares outstanding, and proves out the company’s upside potential in the marketplace.
- Second, the company operates in a specialized field that many investors may resort to placing in their “too hard” pile, leaving what could be substantial returns on the table, even though the business’ value proposition is readily apparent, even to a non-technical audience.
- Third, it’s human nature to want to wait until initial European TeaRx Dry Eye revenue shows up on income statements before making an investment, gauging market reception before putting money to work, even at the expense of missed returns.
These sidelined investors, putting downward pressure on the stock, set up more seasoned allocators to short-circuit human nature and buy into pessimism, recognizing that all publicly available information on DiagnosTear, as we’ve delineated in this article, suggests the company is on the verge of becoming a diagnostic technology leader.
Should operations scale and demonstrate reliable growth, look for the stock to re-rate, facilitating value-added technology acquisitions, as well as the expansion of TeaRx’s horizons to indications beyond ophthalmology, including neurodegenerative diseases, with eyes on leveraging the multitude of biomarkers in our tears towards newfound heights in patient and shareholder value.
Join the discussion: Find out what investors are saying about this medical device stock on the DiagnosTear Technologies Inc. Bullboard and make sure to explore the rest of Stockhouse’s stock forums and message boards.
Stockhouse does not provide investment advice or recommendations. All investment decisions should be made based on your own research and consultation with a registered investment professional. The issuer is solely responsible for the accuracy of the information contained herein.
For full disclaimer information, please click here.





